ST-01156 is our first–in-class, precise glue-based degrader, now in clinics.
ST-01156 is an orally administered, brain-penetrant, selective small-molecule degrader of RBM39 designed to eliminate oncogenic RNA-splicing dependencies across multiple tumor types.
Why RBM39 matters
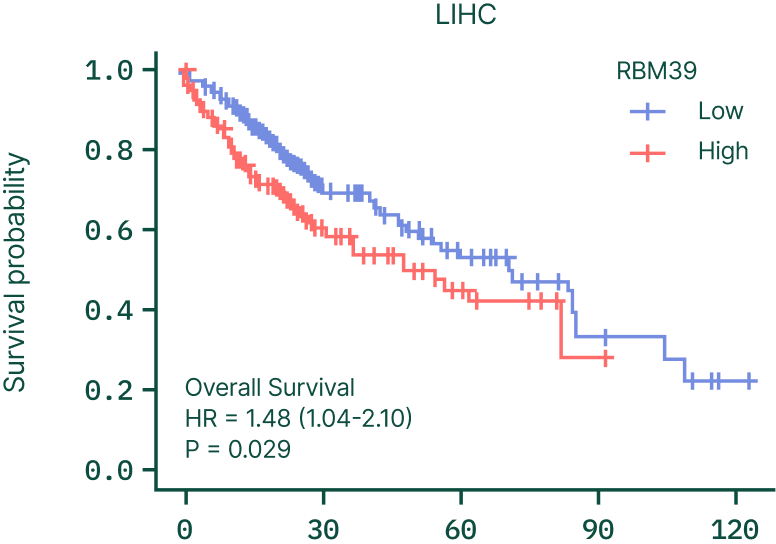
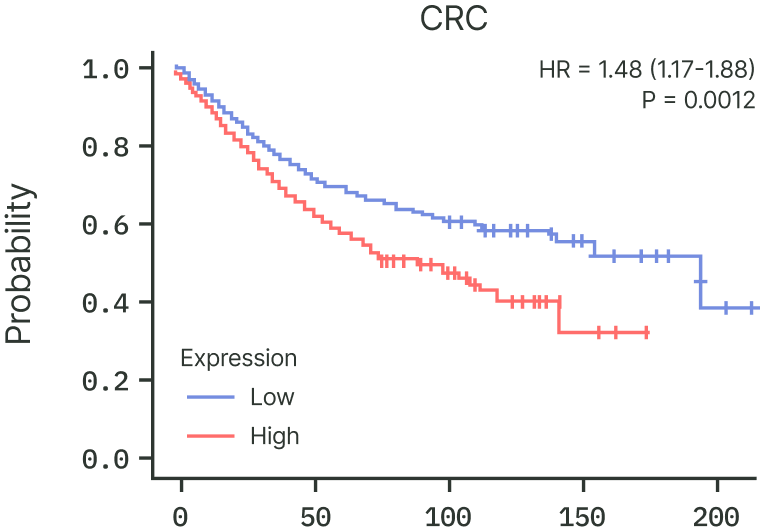
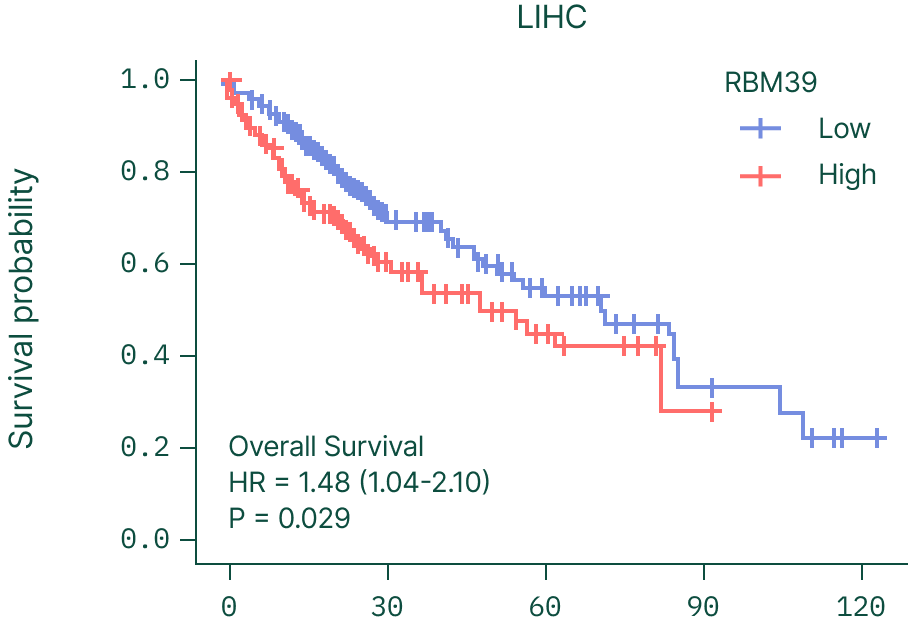
RBM39 is frequently overexpressed in solid tumors, and higher RBM39 levels correlate with significantly worse survival across cancers such as liver and colon (CRC) cancer.
This reinforces RBM39 as a biologically essential, clinically relevant driver of cancers beyond its traditional splicing factor activities.
Degrader programs built on deep biological insight and rigorous clinical translation.
SEED's pipeline is built for real-world impact, targeting diseases where standard modalities fail. Our lead RBM39 degrader (ST-01156) is in Phase 1 trials for Ewing sarcoma, hepatocellular carcinoma and other related tumors; complemented by 6 active programs supported by top clinical and strategic partners
What makes ST-01156 different
Mechanism01
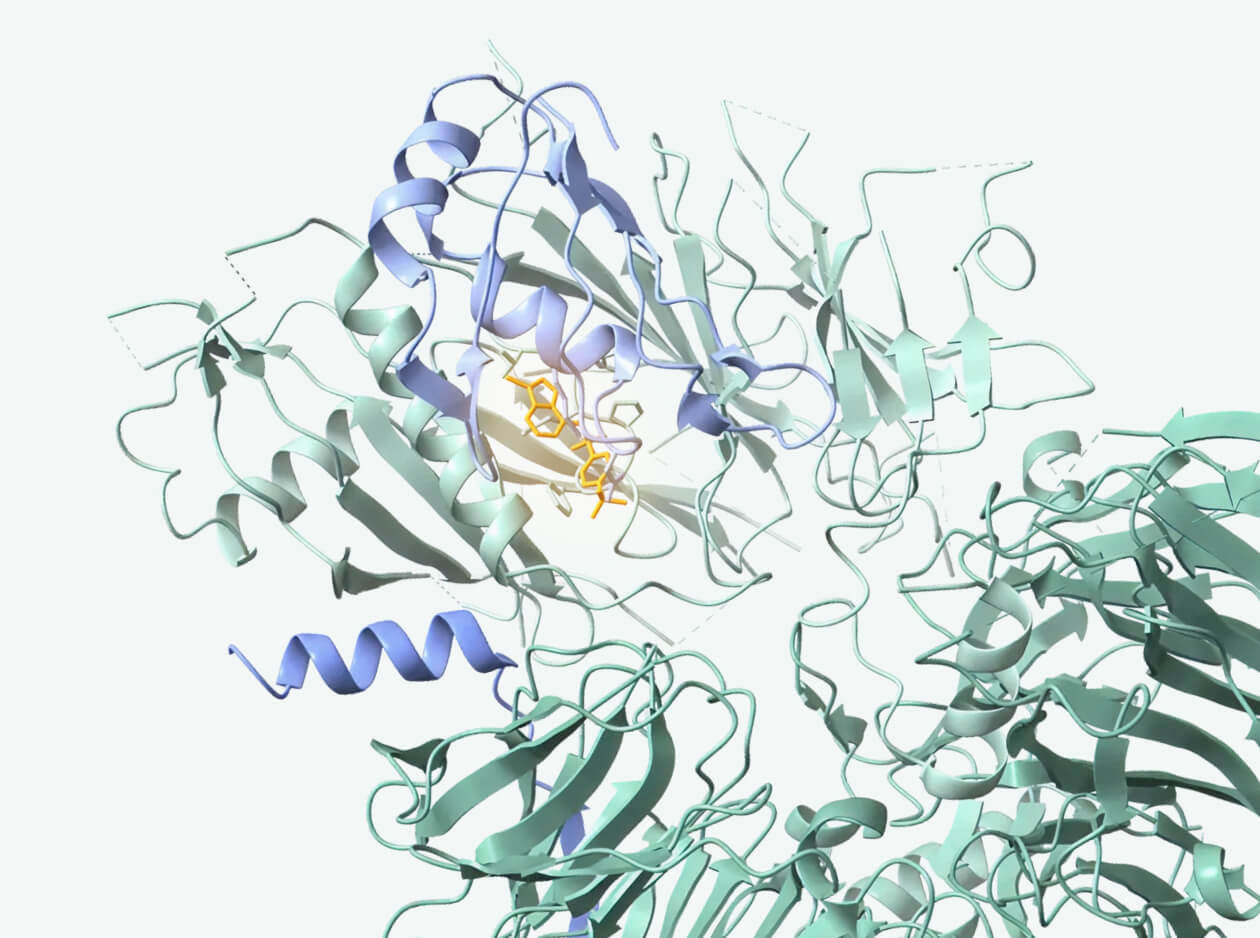
Induces proximity between RBM39 and a novel E3 ligase DCAF15 via SEED’s custom-designed glue molecule.
Selectivity and stability02
Proven preclinical specificity with minimal off-target activity; designed molecule with improved stability.
Applicable to orphan, pediatric
and large cancer indications03
and large cancer indications
Regulatory tailwinds in rare cancers with high unmet need.
Preclinical proof04
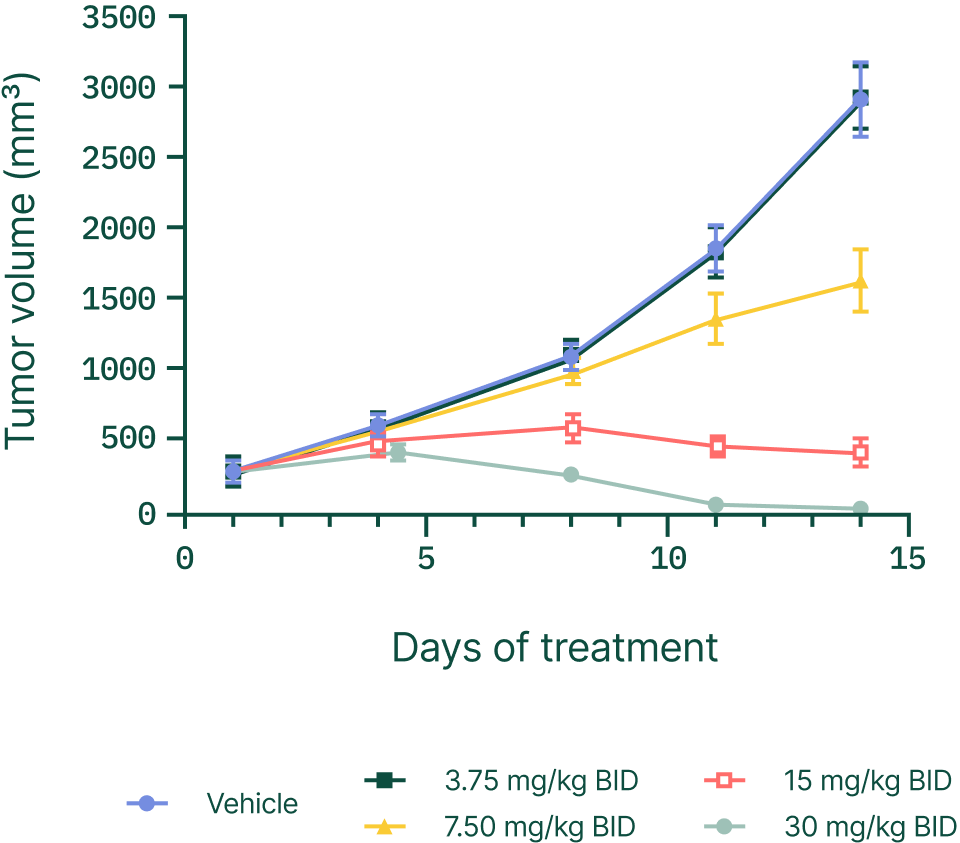
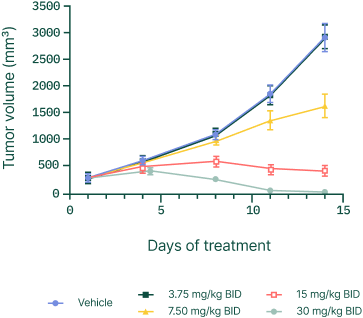
Demonstrated complete tumor regression in xenograft models in a number of cancers.
Starting with Ewing sarcoma.
Expanding across solid tumors.
Ewing sarcoma
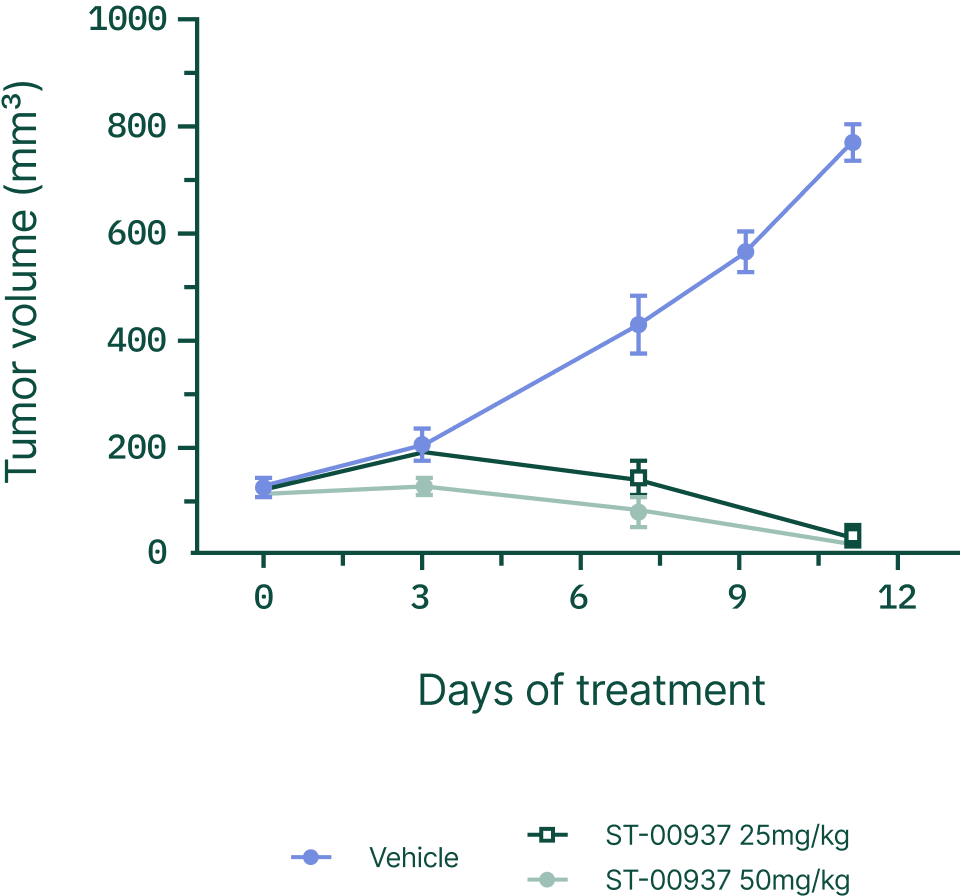
RBM39 dependence has been observed across multiple solid tumors, including neuroblastoma, colon and prostate cancer, and hepatocellular carcinoma, supporting a clear expansion path from rare pediatric cancer into larger oncology indications.
Rare Pediatric and Orphan Cancer designation by US FDA
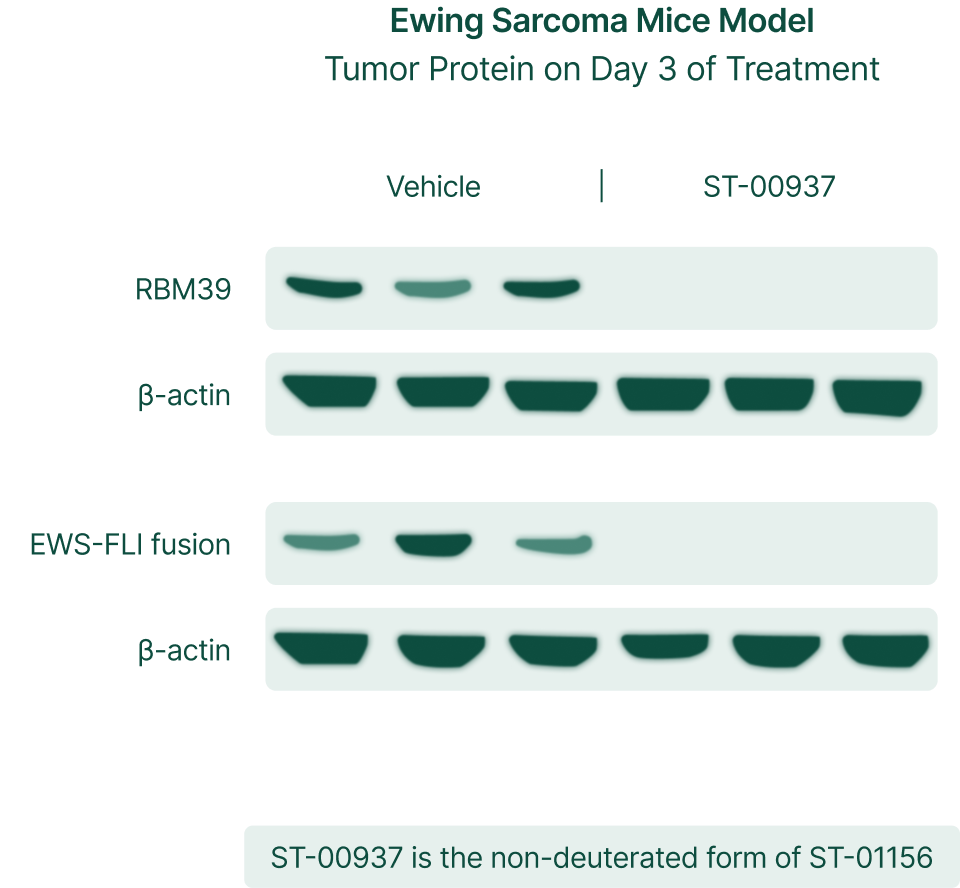
Total elimination of RBM39 and EWS-FLI fusion which causes 90% of Ewing Sarcoma
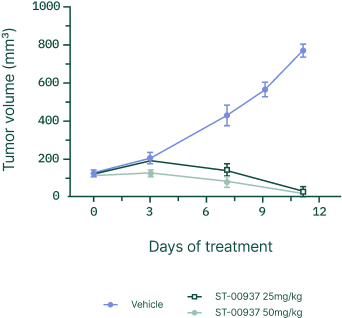
Beyond Ewing sarcoma
RBM39 dependence has been observed across multiple solid tumors, including neuroblastoma, colon and prostate cancer, and hepatocellular carcinoma, supporting a clear expansion path from rare pediatric cancer into larger oncology indications.
Total elimination of RBM39 and EWS-FLI fusion which causes 90% of Ewing Sarcoma
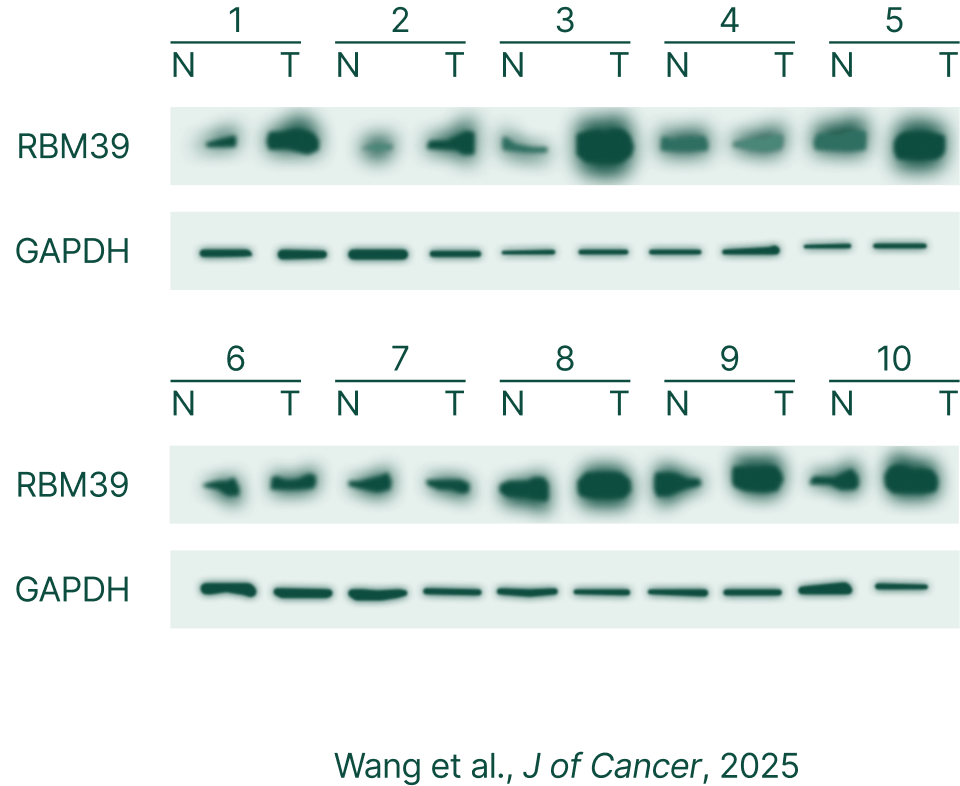
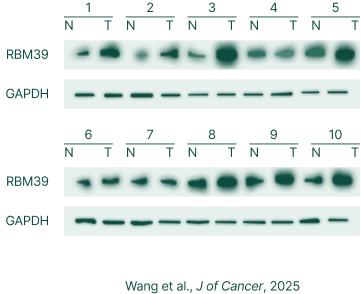
RBM39 high expression in colon cancer, not in normal liver tissue
ST-01156 is guided by leading oncology investigators including Dr. George Demetri at Dana-Farber, Dr. Bob Maki at Memorial Sloan Kettering, and Dr. Gordi Rodon at MD Anderson.



Extending molecular glue
degradation into neurodegeneration.
SEED is advancing an oral molecular-glue degrader targeting pathological Tau for Alzheimer’s disease and related neurodegenerative disorders, addressing a global patient population of more than 50 million.

The science behind our Tau degrader
Pathological Tau aggregation is a core driver of neurodegeneration, disrupting neuronal structure and function. While most approaches attempt to block downstream effects, SEED’s strategy is to directly eliminate Tau at the protein level through targeted degradation.